I’ve recently received several questions regarding what a confocal does that makes it better than a non-confocal fluorescence microscope. I figured it may be helpful to answer this in a post, so:
- Both the confocal and the widefield microscope deliver fluorescent excitation light through the objective lens into the specimen.
- Both the confocal and the widefield microscope have an resolution limited by the objective, NOT the instrument.
- Both the confocal and the widefield microscope won’t avoid or break the laws of resolution limits, like super resolution systems do.
So, what advantage does the confocal have over conventional widefield systems? First, the confocal will remove out of focus light from the image, that the widefield system will not. In practical application, this out of focus light is so signifigant that it effectively reduces the resolution of the widefield system!
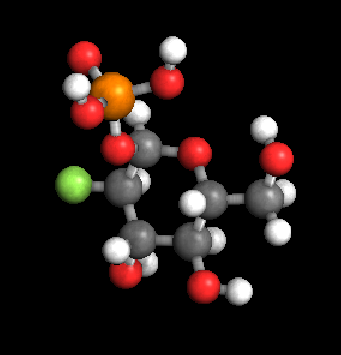
First, let’s look at a typical fluorophore, green fluorescent protein (GFP) at the molecular level:
Note that once an excitation (blue) photon is absorbed by this molecule, and an atomic order is dropped, the emission photon (green) is emitted. This all occurs at the (unresolvable) molecular level.
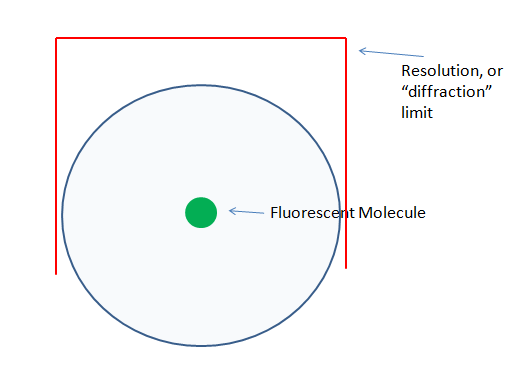
Of course, we typically don’t image one molecule, so this occurs multiple times, across multiple molecules. What we actually “see” in the microscope, is basically a blob, that represents the localization(limited by the resolution of the objective) of this molecule. Here is a (not to scale!) rendering of this affect.
This blob that we collect is a resolution-limited sampling of the molecule’s location. The blue circle represents the emission photons, traveling away from the molecule in all directions.
The core thing to understand here is that all systems (that are non super-resolution), are limited to collecting the diffraction-limited blob (blue circle) we see above. Confocal systems do not increase the resolving power of the instrument!
So far we’ve looked at what positive signal we collect. In any instrument we also have to consider the noise sources, this is really where the confocal operates. In a widefield system we see an image that includes:
– Specimen Noise (light distortions caused by the specimen container or the specimen itself, or auto-fluorescence in the specimen)
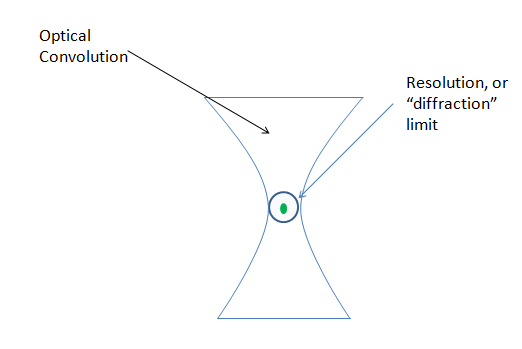
– Out of focus light (optical convolution) caused by the increasingly focused light hitting and emitting from the sample.
– Other errors caused by the optical train and the detector (camera).
Of these, the confocal eliminates, or rather reduces, the optical convolution noted above, but does not affect the specimen auto-fluorescence, or the optic/detection noise.
If we view the optical convolution in Z, we see a shape that looks like the one below. This is referred to as a PSF, or Point Spread Function. This shape indicates the spread of light, from a point of focus, above and below the focal plane:
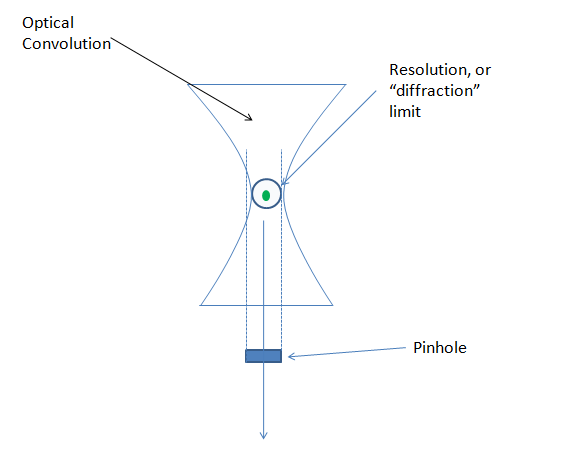
The confocal operates on the principle that a pinhole (small aperture) will block non-focal light from passing through it. Employed in a microscope, the effect is that our optical convolution is reduced to a point where it is effectively eliminated. In the example below you’ll note that this is most effective on the sides of our molecule, but not on the top or bottom. Overall the pinhole is quite helpful in reducing the noise that interferes with positive signal in any focal plane.
A better example of this can be found on wikipedia’s explanation of a point spread function:

So, by removing the convolution from the iamge, what is rendered in a single focal plane is an image of our signal blob, that has the majority of the optical convolution removed. An example between the two (A=widefield and b=confocal) is shown below:

Now the single pinhole doesn’t explain how this pinhole sits in the lightpath, or how an image is formed. There are two options used for applying a pinhole to an image, and these options form the difference between Laser Scanning and Spinning Disk confocals.(See links at left for info on each method)
Ultimately, each method has pluses and minuses. You’ll find that almost every major research facility has one or both types installed, and being familiar with the use of each is a handy skill to have.
So, a confocal will remove the out of focus light from a specimen and will reduce the overall excitation energy needed to form a good image. This results in the following advantages over widefield:
- Lower excitation energy used = healthier for live cells
- Reduction/elimination of background out of focus light reveals much cleaner z slices
- Increase in z slice clarity = more accurate imaging of 3-d structures
There are other technique specific advantages to confocals as well, but these are the big points. In later posts I’ll explain how super resolution systems build upon this and other technology, to avoid the laws of resolution limits and create amazingly clear images!
– Austin

Comments
One response to “Confocal vs. Widefield”
[…] course, one simple solution, but an expensive one, to this problem is to add a pinhole-based confocal to the optical system. While a confocal does reduce or eliminate the out of focus haze in the […]